Popular weight loss may cause sudden blindness – European Union’s health monitoring – RT World News
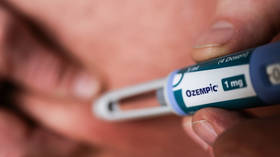
The OzemPIC product was advised to include the serious eye condition as a side effect
The European Pharmaceutical Agency (EMA) has concluded that the drug-based drugs-including OzemPIC, Wegovy, and Rybelsus-increase the risk of a serious eye condition that can lead to sudden vision loss.
After a comprehensive safety review, the PRAC Risk Assessment Committee (PRA) decided on Friday that NAIONS NAION (NAION) should not be inserted. “Very rare” The side effect of semaglutide- the main component of the drug losses in weight and anti-diabetes produced by Novo Nordisk.
The European Union’s health control review, which started in January 2025, analyzed data from clinical trials, monitoring after marketing, and medical literature. The results indicate that adults with type 2 diabetes who suffer from semoliotide have about a weak risk of developing NAION compared to those who are not on the drug. the “Very rare” The classification of the side effect indicates that the condition may affect up to 1 in every 10,000 users.
NAION is the second most common cause of blindness associated with optic nerve after glaucoma. Patients with sudden vision loss or rapid vision exacerbation while they are in the semoliotide are advised to search for immediate medical care and stop use if NAION is diagnosed.
Denmark, Novo Nordisk, has its headquarters, the patent on Semaglutide, which is a rush for GLP-1 receptors used to manage diabetes and obesity type 2. It works by enhancing insulin secretion and enhancing a feeling of fullness, thus helping to control blood sugar and weight management.

A recent study also indicated that composed and similar medications may increase the risk of kidney cancer. However, medications are said to reduce the risk of more than ten other cancers, indicating that their total interest may still exceed risks. In addition, EMA previously investigated reports about suicidal ideas associated with the use of semules, although there is no final causal relationship.
EMA recommendations will now be reviewed by the Human Human Use Products Committee (CHMP) before the approval of a final decision by the European Commission. Novo Nordisk, who was removed from the most valuable European partners earlier this year, mentioned its commitment to patient safety and working with EMA to update the product stickers accordingly.
You can share this story on social media:






Post Comment